 The Phase Change Matters e-mail newsletter is a weekly summary of the latest news and research on phase change materials and thermal energy storage. To subscribe, visit www.puretemp.com/subscribe. For more frequent updates, follow @puretemp on Twitter or visit the Phase Change Matters blog, www.puretemp.com/pcmatters.
The Phase Change Matters e-mail newsletter is a weekly summary of the latest news and research on phase change materials and thermal energy storage. To subscribe, visit www.puretemp.com/subscribe. For more frequent updates, follow @puretemp on Twitter or visit the Phase Change Matters blog, www.puretemp.com/pcmatters.
ENERGY STORAGE
Finnish team’s PCM is designed to lock in solar, waste heat for later use
Researchers in Finland are developing a novel phase change material that combines sugar alcohols and sodium polyacrylate, the superabsorbent polymer used in disposable diapers. The material is designed to store solar heat collected in summer and release it for use in winter. The material could also be used to store industrial waste heat.
Team HeatStock, whose members include chemists, energy engineers and physicists from three universities, is one of 20 semifinalists in the 2017 Helsinki Challenge. Finalists, to be selected in June, will compete for a share of 375,000 euros in research funds. The winners will be announced in December.
 Team HeatStock presented its technology at the Helsinki Challenge pitch night last month.
Team HeatStock presented its technology at the Helsinki Challenge pitch night last month.
“The charging of our storage happens by melting the active material of our solution,” said Aalto University research scientist Salla Puupponen. “However, when the melt material starts to cool, it doesn’t release the heat on crystallization as conventional phase change materials, but instead we can keep our material as low temperatures as we want, as long time as we want without losing the stored energy.”
In an interview with Phase Change Matters, team leader Ari Seppälä, a senior scientist at Aalto, describes the technology in further detail.
Q: On the Helsinki Challenge website, you mention that the material will be used to store heat from “solar collectors.” That’s solar thermal, not photovoltaic, correct?
A: Yes, that meant solar thermal collectors. But that is just a one possibility. Other options include such as storing waste heat from industrial processes and storing the surplus heat produced by CHPs (combined heat and power plants) during summertime. As CHPs are often linked with district heating systems (at least in Nordic countries) delivering hot water to residents, the surplus heat could also be exploited for charging the storages of residential buildings during summer for wintertime use.
Q: How is the project being funded now?
A: We have Aalto University strategic funding (Aalto Energy Efficiency Program) and also funding from Fortum Foundation. However, our funding ends during this year. Currently we are looking for new funding possibilities. We are also looking for more collaborators and community members for the research and the competition. So, experts, scientists, companies and organizations who are interested in our research are most welcome to join us!
Q: Your PCM sounds like a composite. What are its components and how are they combined?
A: Our PCM can be classified more likely as a mixture than as a composite. It is composed of a polyol in a cross-linked polyelectrolyte matrix.
Q: What is the PCM’s melting point?
A: The melting point is about 100º C.
Q: What is the PCM’s thermal energy capacity in joules per gram?
A: The heat of melting is 180-280 J/g depending on the composition. The heat of crystallization of the material is currently approximately 140-170 J/g. We aim at developing the latent heat of our material further.
Q. You have describe the material as having “phase-change properties that had never been seen before with any material.” What are those properties?
A: Operation of our novel material is based on so-called cold-crystallization, in which the conventional melt-crystallization on cooling is prevented and the material crystallizes only on heating. Supercooled PCM does not seem to crystallize even with a seed crystal below the cold-crystallization temperature. Anomalously, the PCM seems to be stable also above the glass-transition temperature. The novel operation principle enables long-term storing of thermal energy, and discharge of the storage by a small heat pulse.
Cold-crystallization is previously observed also for hydrated polymers, in which water is absorbed by hydrophilic polymers. However, in these cases the amount of cold-crystallizing water is small and the crystallization properties are not conserved in the repeated melting-crystallization cycles. Our material instead can consist up to 90 percent of actual PCM and can be cycled without notable changes in phase change properties.
In addition, the cold-crystallization temperature can be adjusted by the changing the material composition.
Q: What are the key steps in your scale-up plans?
In the beginning, we aim at scaling-up our sample size from tens of milligrams to a kilogram scale. In the scale-up, it is crucial that the material properties, especially the stability of supercooled state, remain unaltered. That is of course an open question, as it is well known that the stability of metastable states decreases with increasing volume of the sample. However, our small, deeply supercooled samples did not crystallize even with seeds and thus the operation of our material differs substantially from conventional materials. After the scale-up process, we will study the triggering of the crystallization by the heat pulse. We also aim at building a practical demo linked with a heat loading and releasing system.
We will later also look for creating similarly behaving materials based on different PCMs.
Q: Have you published research papers on the material?
A: There are no published papers concerning this new material so far. The manuscript on this material, (Puupponen and Seppälä, Cold-crystallization of polyelectrolyte absorbed polyol for long-term storing of thermal energy) has just been submitted for review and a patent application is pending.
Here are links to recent journal papers related to our other PCM studies:
PCM for long-term storage:
Puupponen S, Mikkola V, Ala-Nissilä T, Seppälä A, (2016) Novel microstructured polyol–polystyrene composites for seasonal heat storage, Applied Energy 172 96–106.
Thermodynamics of solidification and melting:
Seppälä A., Irreversibility of solidification and of a cyclic solidification-melting process, (2012), International Journal of Heat and Mass Transfer, 55 1582-1595.
Heat transfer nanofluids with PCM particles:
Puupponen, S., Seppälä, A., Vartia, O., Saari, K., Ala-Nissilä, T., Preparation of paraffin and fatty acidphase changing nanoemulsions for heat transfer (2015), Thermochimica Acta, 601, 33-38
Mikkola V, Puupponen S, Saari K, Ala-Nissila T, Seppälä A., Thermal properties and convective heat transfer of phase changing paraffin nanofluids, (2017), accepted for publication in International Journal of Thermal Sciences.
PATENTS
Cushion with phase change material containers
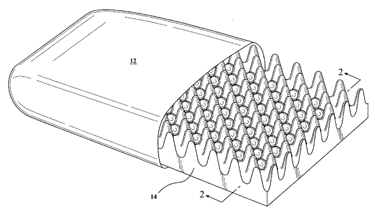 U.S. patent application 20170055719 (applicant Bob Jacquart, Ironwood, Mich.):
U.S. patent application 20170055719 (applicant Bob Jacquart, Ironwood, Mich.):
“A cushion comprising a body of cushioning material having a first surface and a second surface opposing the first surface. The first surface has a plurality of upwardly extending fingers and a plurality of spaced apart pockets defined between the fingers. The cushion has a plurality of phase change material (PCM) containers each containing a phase change material, each of the phase change material (PCM) containers being disposed in one of the pockets in the body of cushioning material.”
IN BRIEF
• Global chemicals production started the first quarter on a strong note, according to the American Chemistry Council. The council’s Global Chemical Production Regional Index showed that that headline global production rose 0.6 percent in January after a similar gain in December.
• Packaging giant Sonoco has again made Fortune magazine’s list of most-admired companies, ranking second in the packaging/containers category.
• Alexium International has appointed Dirk Van Hyning as CEO, effective June 30. He will replace Nicholas Clark, who remains on the board and assumes a new role as executive director strategy. Former U.S. congresswoman Karen Thurman has joined Alexium’s board as a non-executive director.
• Submissions for the 2017 Reaxys PhD Prize close on March 13. The international competition is open to Ph.D. students or recent graduates conducting innovative research in synthetic chemistry. Forty-five finalists will present their research at the Reaxys Prize Symposium in Shanghai in October. Three winners will be selected, with each earning a $2,000 award.
• In an interview with Energy Storage Report, Ice Energy CEO Mike Hopkins says his company is looking to tie up partnerships with solar installers in regions where net metering is being phased out.
• Carnegie Mellon researchers have developed a thermally conductive rubber material that they’ve nicknamed “thubber.” The new material, which can stretch more than six times its initial length, is an electrically insulating composite. Potential applications include soft robotics and athletic wear.
• New from Wise Guy Reports: “Global Thermal Energy Storage (TES) Market Research Report 2017“
• Sunamp Ltd. is teaming up with Glasgow University and partners in China to boost the performance of Organic Rankine Cycle power plants that use renewable heat sources for distributed heat and power supply in China. The joint project has been awarded 2 million pounds in funding from the China-UK Research and Innovation Bridges program. Sunamp’s PCM heat batteries will be integrated with ORC plants to store heat energy for power generation when the sun doesn’t shine.
RESEARCH ROUNDUP
For our full list of recent academic research, see puretemp.com/academic. Here are highlights from the past week:
From Energy and Buildings:• Thermal effects of a novel phase change material (PCM) plaster under different insulation and heating scenarios
From Materials Letters:
• Form-stable phase change material embedded with chitosan-derived carbon aerogel
From Renewable Energy:
• Investigation of Unbranched, Saturated, Carboxylic Esters as Phase Change Materials
From Energy Conversion and Management:
• Intensification of monostearin (phase change material) synthesis in infrared radiated rotating reactor: Optimization and heterogeneous kinetics
From Colloids and Surfaces A: Physicochemical and Engineering Aspects:
• Synthesis of epoxy-loaded poly(melamine-formaldehyde) microcapsules: effect of pH regulation method and emulsifier selection
From Materials Chemistry Frontiers:
• Dual-encapsulation of octadecanol in thermal/electric conductor for enhanced thermoconductivity and efficient energy storage
From Applied Thermal Engineering:
• Lattice Boltzmann modeling of melting of phase change materials in porous media with conducting fins
• Preparation research of novel composite phase change materials based on sodium acetate trihydrate
• The quasi-enthalpy based lattice Boltzmann model for solid-liquid phase change
From AIP Conference Proceedings:
• A TRNSYS simulation of a solar-driven ejector air conditioning system with an integrated PCM cold storage
• A simulation study of a solar collector using phase change materials for air heating application needs
NETWORKING
Connect with PCM experts and industry leaders on LinkedIn
More than a thousand of your peers have joined a LinkedIn group devoted to the discussion of phase change material and thermal energy storage. The Phase Change Matters group is an interactive complement to the award-winning blog and newsletter of the same name.
 You are invited to join the group and connect with PCM and TES experts from around the world. New members include Dr. Jonathan Fagerström, R&D engineer, Finland; Yash Doshi, senior research associate, Allied Analytics, India; Lauren Mannix, building efficiency intern at Johnson Controls, Tarboro, N.C.; and Kevin Hrebenar, president and COO, Dooley Chemicals, Chattanooga, Tenn.
You are invited to join the group and connect with PCM and TES experts from around the world. New members include Dr. Jonathan Fagerström, R&D engineer, Finland; Yash Doshi, senior research associate, Allied Analytics, India; Lauren Mannix, building efficiency intern at Johnson Controls, Tarboro, N.C.; and Kevin Hrebenar, president and COO, Dooley Chemicals, Chattanooga, Tenn.